Working of Water Softner
Water softeners are specific ion exchangers that are designed to remove ions, which are positively charged. Water Softeners mainly move calcium (Ca2+) and magnesium (Mg2+) ions.
A water softener collects hardness minerals and flushes them away to drain. Adsorption reaction takes place over the surface of resin. As the hard water pass through it, the resin adsorbs calcium and magnesium ions and gives sodium ions which do not constitute to hardness. The impurity is present in the form of molecules such as MgCl2, CaSO4, MgSo4 etc.
The water softener consists of a pressure vessel containing cation exchange resin. The Cation resin has Sodium (Na+) Charge on it. The Cations in water Ca++, Mg++ pass through the resin where they are chemically exchanged for Sodium (Na+) ion. The reaction-taking place in softener is shown as :
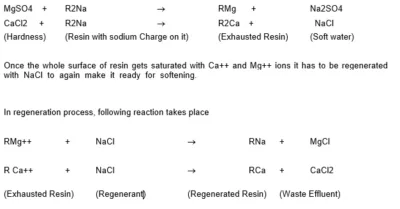
Raw water should not be taken into the Boiler. Use of raw water will give rise to scaling and carry over in the boiler. Only treated water should be used for makeup purposes. It is preferable to use de mineralized water for the same. However for low pressure boilers softened water may also be used.
In either case, while installing the treatment plant, care should be taken to see that adequate capacity is provided. Also adequate storage capacity for the treated water should be planned.
